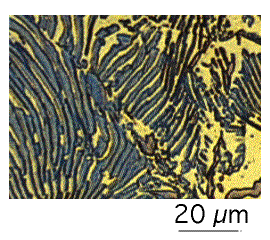

A colony of pearlite is a bicrystal of cementite and ferrite. Suppose we represent the cementite by a cabbage and the ferrite by a bucket of water. Placing the cabbage in the water properly represents the pearlite colony. The cementite is a connected single crystal in three dimensions, and the ferrite is similarly a single crystal in three dimensions.
When examined on a planar section, pearlite appears to consist of alternating layers of ferrite and cementite, rather like sectioning a cabbage with a knife.
Pearlite forms by the solid-state transformation of austenite. The cementite and ferrite grow cooperatively at a common transformation front. In plain carbon steels (Fe-C) alloys, the average composition of the pearlite is identical to that of the austenite from which it grows. The ferrite, cementite and austenite can then exist in equilibrium at a unique temperature, the eutectoid temperature. In alloy steels (e.g. Fe-Mn-C), the three phase equilibrium can occur over a range of temperatures, so that pearlite can exist in equilibrium with austenite.
 |
 |
| Two-dimensional morphology of pearlite, apparently consisting of alternating layers of cementite and ferrite. | Three-dimensional analogy to the morphology of pearlite, i.e. the cabbage represents a single crystal of pearlite, and the water in the bucket the single crystal of ferrite. |
 |
| Transmission electron micrograph of tempered martensite in an Fe-C-Mo steel. The carbides are (Fe,Mo)2C. |
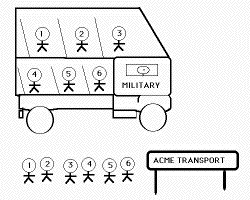
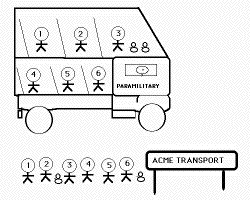
Most solid--state transformations fall into two categories. Displacive transformations involve a coordinated motion of atoms as the parent lattice is deformed into that of the product. There is no diffusion and hence there exists an atomic correspondence between the parent and product phases. A numbered sequence of atoms is maintained in the product phase. Such transformations are called military transformations because there is a disciplined transfer of atoms; the analogy here is that of a highly disciplined queue of soldiers ordered to board a military bus. The number sequence of the bus is identical to that in the queue. The soldiers do not have a choice as to their neighbours (analogous to the solute-trapping phenomenon). The situation is not at equilibrium.
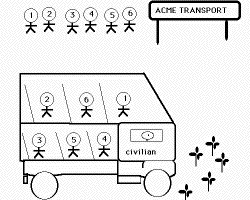
It is the diffusion of atoms that leads to the new crystal structure during a reconstructive transformation. The flow of matter is sufficient to avoid strains and solutes may partition between the parent and product phases. The diffusion--driven flow of matter destroys any atomic correspondence between the parent and product phases. This is analogous to a numbered queue of civilians who board the bus in a disorderely manner, so that the the sequence in the queue bears no resemblance to that in the bus.
A paramilitary is a partly disciplined force. A paramilitary transformation is one in which interstitial atoms (which can move rapidly) partition during transformation but the change in crystal structure is achieved by displacive transformation. This is a common mechanism of transformation in Fe-C and V-H alloys. The interstitials thus achieve equilibrium subject to the constraint that the substitutional atoms do not diffuse.
The images below can be enlarged by clicking on the thumbnails.
 |
 |
 |
| Military Transformation | Civilian Transformation | Paramilitary Transformation |
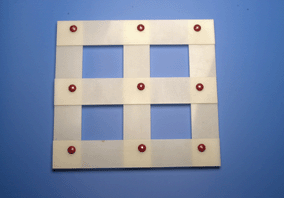
Substitutional atoms do not diffuse during a displacive transformation. The pattern in which the atoms are arranged neverthless changes, leading to a macroscopic change in the shape of the transforming region. This model illustrates the mechanism of a displacive transformation, in which there is a coordinated motion of atoms.
Quicktime movies showing the deformation process: Movie 1 (200 kBytes); Movie 2 (26 MBytes).
MPG movies showing the deformation process: Movie 3 (200 kBytes)
 |
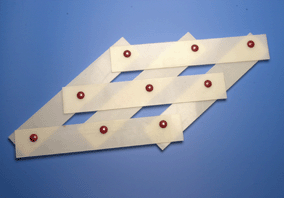 |
| Starting shape and crystal structure. | Final shape and crystal structure following a homogeneous deformation which is a shear. |
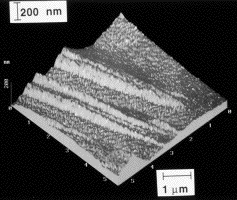 |
| During a displacive transformation, the change in crystal structure is achieved by a homogeneous deformation of the parent phase. A sample which is polished flat and then transformed to bainite will therefore exhibit displacements on the free surface. This atomic-force microscope image shows the displacements. |
 |
 |
| A glass vial containing a liquid that foams. Shaking results in a fine foam, which slowly coarsens with time. The coarsening process is somewhat analogous to grain growth in solids. | The same vial, after allowing some time for the foam to coarsen. The process occurs in order to reduce the surface per unit volume. |
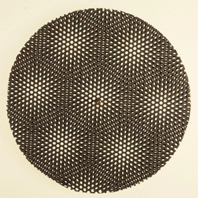
A coincidence-site lattice is constructed by taking two lattices with a common orgin, and allowing them to pentrate all space; coincidence points are those lattice points common to the two lattices. This model shown below reproduces this effect in two dimensions. Two circular grids connected at the centre. The grids are first aligned to indicate a single crystal. Rotating one with respect to the other produces a variety of coincidence site lattices, even when the angle of rotation is large. This model is place on an overhead projector. The effect can also be seen on movies:
Quicktime movies showing the deformation process: Movie 1 (200 kBytes); Movie 2 (6 MBytes).
MPG movies showing the deformation process: Movie 3 (200 kBytes)
 |
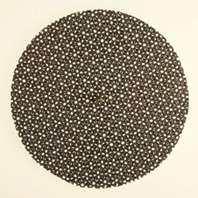 |
| The pattern shows coincidence points between the two grids which are rotated with respect to each other. | The pattern shows coincidence points between the two grids which are rotated with respect to each other. |
 |
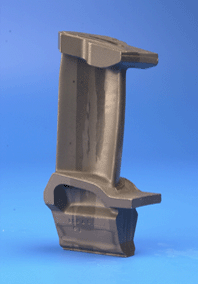 |
| The blades are made out of a nickel-base superalloy with a microstructure containing about 65% of gamma-prime precipitates in a polycrystalline gamma matrix. The creep life of the blades is limited by the grain boundaries which are easy diffusion paths. | The blade is made out of a nickel-base superalloy with a microstructure containing about 65% of gamma-prime precipitates in a polycrystalline gamma matrix. It has been directionally-solidified, resulting in a columnar grain structure which mitigates grain-boundary induced creep. |
 |
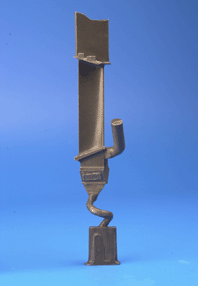 |
| The blade is made out of a nickel-base superalloy with a microstructure containing about 65% of gamma-prime precipitates in a polycrystalline gamma matrix. It has been directionally-solidified, resulting in a columnar grain structure which mitigates grain-boundary induced creep. | The blade is made out of a nickel-base superalloy with a microstructure containing about 65% of gamma-prime precipitates in a single-crystal gamma matrix. The blade is directionally-solidified via a spiral selector, which permits only one crystal to grow into the blade. |
 |
 |
| Photograph illustrating the very large size of a modern jet engine on a civil aircraft. Other similar photographs: Image 1 Image 2 | Photograph of a compete airliner. Many more pictures of jet engines. |
The microstructure consists of a mixture of delta, an ordered intermetallic compound Al3Li with a cubic-P crystal structure, whereas the matrix is a disordered solid solution with a cubic-F crystal structure. The two phases are in cube-cube orientation with coherent interfaces.
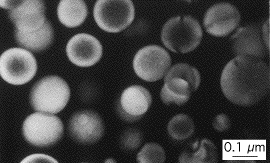 |
| Aluminium-lithium alloy with delta-phase particles |
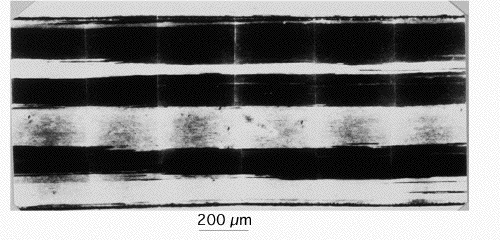 |
| Recrystallisation generally leads to a final microstructure of equiaxed grains. Recrystallisation in a temperature gradient can lead to a coarse, columnar grain microstructure. This is common in oxide dispersion-strengthened nickel superalloys of the type illustrated here. |
This is a macrograph of an aluminium ingot (about 5 cm wide) made by casting commercially pure aluminium in a metal mould. The liquid in contact with the mould wall solidifies relatively rapidly, giving a fine, equiaxed grain structure there. This zone is called the 'chill zone'. The grains then start to grow towards the centre, in the general direction of maximum heat flow, giving a columnar-grain structure. Those grains which have their maximum growth directions most parallel to the heat flow stifle the growth of less favourably oriented grains, leading to a coarsening of the columnar structure as solidification progresses towards the centre.
There is sometimes a region of equiaxed grains in the centre of the ingot, arising from solid particles that form at the liquid surface, or from fragments of metal solidifying from the mould wall.
The central pipe is due to the contraction of the liquid as it solidifies.
Higher resolution images (0.5 Mbytes) of ingots are also available:
|
|
| PT Group Home | Materials Algorithms |

|

|