Crystal structure of alpha-plutonium
Crystal structure of alpha-plutonium
Crystal structure of alpha-plutonium
Crystal structure of alpha-plutonium
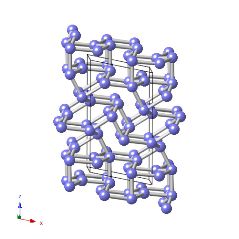
Crystal structure of alpha-plutonium

Plutonium is a strange metal with a rather low melting temperature of only 913 K. Like water, the first phase that forms during the solidification is less dense than the liquid. As a consequence, it undergoes a series of solid-state transformations which lead to densification. Many of these transformations occur by a martensitic mechanism. Almost all of these phases have low-symmetry crystal structures. Alloying elements such as aluminium are used to stabilise the face-centred cubic form.
The report below describes a model for estimating the lattice parameter of Pt-Al alloys as a function of concentration and temperature.
 Crystal structure of alpha-plutonium |
 Crystal structure of alpha-plutonium |
 Crystal structure of alpha-plutonium |
 Crystal structure of alpha-plutonium |
 Crystal structure of alpha-plutonium |
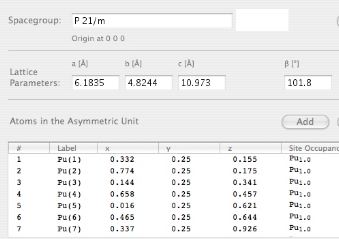
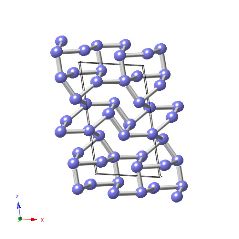
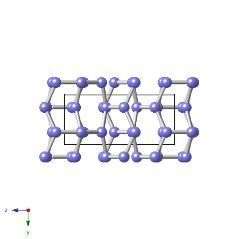
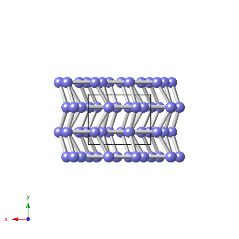
β-plutonium is monoclinic with space group I2/m and lattice parameters a=0.9284 nm, b=1.046 nm, c=7.859 nm, β=92.13° [W. H. Zachariasen and F. H. Ellinger, Acta Crystallographica, 16, 1963, 369].
The structure illustrated below is for pure β-plutonium, but the atoms are coloured differently to indicate the seven types present in the structure.
Crystalmaker file for β-plutonium
| Superalloys | Titanium | Bainite | Martensite | Widmanstätten ferrite |
| Cast iron | Welding | Allotriomorphic ferrite | Movies | Slides |
| Neural Networks | Pearlite | Recrystallisation | Theses | Crystallography |
| PT Group Home | Materials Algorithms |