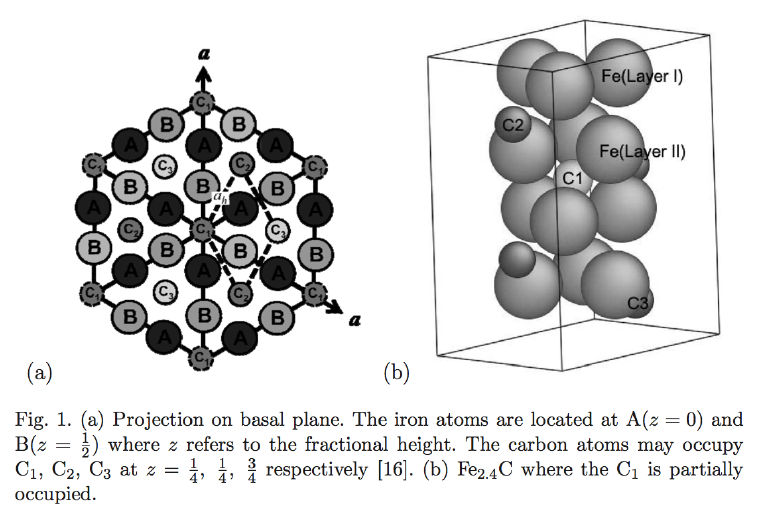

ε-carbide is a transition iron carbide with a chemical formula between Fe2C and Fe3C. It has a hexagonal close-packed arrangement of iron atoms with carbon atoms located in the ocatahderal interstices.
The space group of ε-carbide is P6322. The lattice parameters (Fig. 1) are a = 0.4767 nm and c = 0.4353 nm. The illustrated cell contains six iron atoms and a full complement of carbon atoms. To achieve a composition Fe2.4C the site labelled C1 would on average need to be partially occupied.
[Nagakura, Journal of the Physical Society of Japan 14, 1959, 186-195]
There is some confusion in the literature, where the space group of ε-carbide is taken to be that for hexagonal iron, i.e., P63/mmc. This is illustrated as the smaller unit cell defined by the dashed lines in Fig. 1, with ah = 0.2752 nm. Here a = √3 ah.

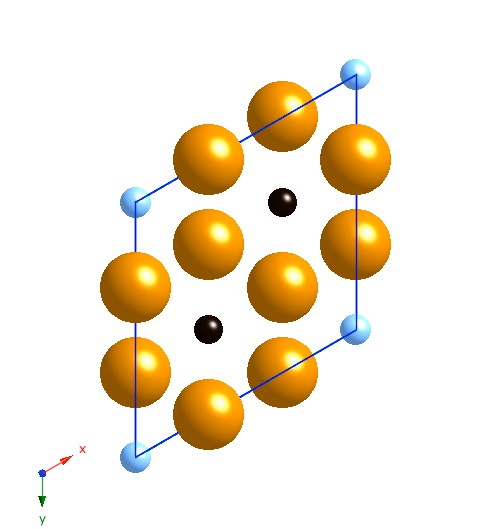 Projection along [001]. The small atoms are carbon, the large ones iron. The black carbon atoms are occupied sites, the blue are partially occupied sites. |
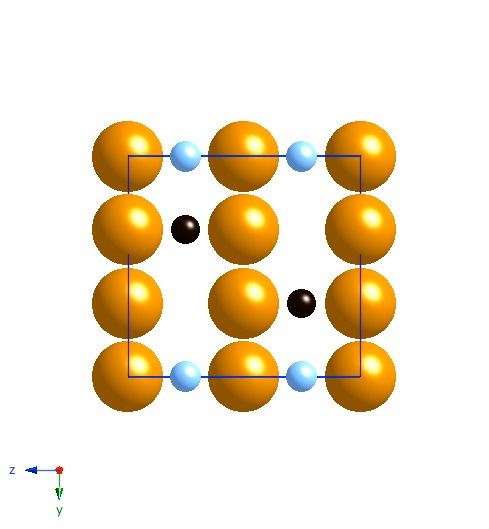 Projection along [100]. The small atoms are carbon, the large ones iron. The black carbon atoms are occupied sites, the blue are partially occupied sites. |
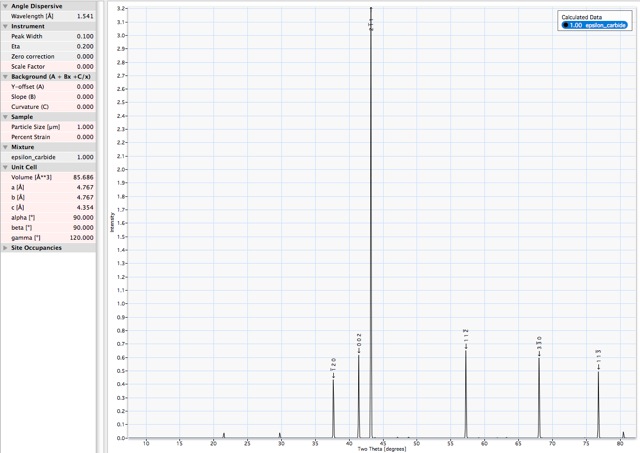 X-ray powder diffraction pattern. Corresponding data and list of d-spacings. |
 Electron diffraction pattern, [001] zone axis |
 Electron diffraction pattern, [010] zone axis |
 Electron diffraction pattern, [100] zone axis |
 Electron diffraction pattern, [110] zone axis. Notice that the [100] and [110] (and [010]) directions are crystallographically equivalent. |
 Electron diffraction pattern, [101] zone axis |
 Electron diffraction pattern, [011] zone axis |
 Electron diffraction pattern, [111] zone axis |
| Superalloys | Titanium | Bainite | Martensite | Widmanstätten ferrite |
| Cast iron | Welding | Allotriomorphic ferrite | Movies | Slides |
| Neural Networks | Creep | Mechanicallly Alloyed | Theses | Retained Austenite |
| PT Group Home | Materials Algorithms |