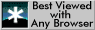
Many compounds with the general formula ABO3 (where A is typically a large divalent cation and B is a smaller 4+ cation) crystallise with the perovskite structure (isostructural with the mineral perovskite, CaTiO3). They form a technologically important class of compounds, finding applications such as ferroelectric sound transducers and heat sensors. MgSiO3 perovskite is believed to be the predominant phase in the Earth's lower mantle, and as such is the most prevalent terrestrial silicate. One reason that so many different cations can be accommodated within the structure is that it is able to adapt by small adjustments of interatomic bond lengths and bond angles. These lead to small distortions of the "ideal" structure (the aristotype) and result in lower symmetry polymorphs. It is these distortions that we shall consider first. In the second and third parts of the demonstration we investigate how such distortions affect physical properties, which may be observed by experiment.
1. Distortions in the perovskite structure
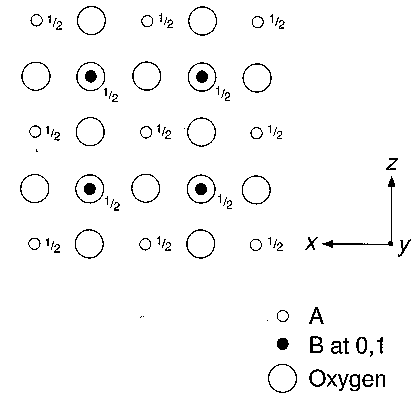
1.1 The ideal perovskite structure, projected onto (010), is illustrated in Fig. 1. Identify the unit cell with its origin at a B atom. Indicate, in a distinctive colour, the mirror planes normal to the paper, and the rotation axes parallel to [010]. Also record the fractional y coordinates of the mirror planes parallel to (010).
1.2 Examine a ball-and-spoke model of perovskite and identify the A and B atoms and the rotation axes parallel to [111]. What is the crystal system of this structure? Does it possess a centre of symmetry?
1.3 An alternative way of visualising the structure is in terms of coordination polyhedra. What is the coordination polyhedron around the B atoms? How are these polyhedra connected in the three-dimensional structure?
1.4 Compared to this ideal structure, many real perovskites are distorted. For example, if the B cation is too small it is displaced from the centre of its coordination polyhedron, towards one of the surrounding oxygen atoms. Fig. 2 shows all the B cations displaced along [001] towards the nearest oxygen. Mark on the symmetry of this distorted structure. Which symmetry elements have been lost? (Note that the symmetry parallel to [001] is unchanged.) What is the new crystal system? Does it possess a centre of symmetry?

Figure 1
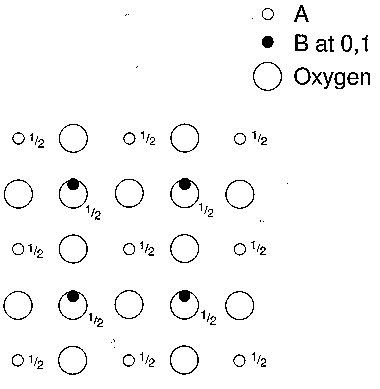
Figure 2
2. Direct observation of the phase transition in BaTiO3 using a heating stage on a polarising microscope
Above 120 °C barium titanate (BaTiO3) has the ideal cubic perovskite structure. Below this temperature Ti moves off the centre of the TiO6 octahedron, reducing the symmetry to tetragonal. This type of change in symmetry of a crystal, which is due to small displacements of atoms off special positions (such as the centre of the octahedron), is called a displacive phase transition. It is possible to observe the transition by using a heating stage on a polarising microscope, since the high-temperature phase (cubic) is isotropic and the low-temperature (tetragonal) phase is uniaxial and optically negative with appreciable optical birefringence. In the transformation from the high-temperature state, three orientations of the tetragonal c axis are possible. The three orientations are illustrated schematically in Fig. 3.
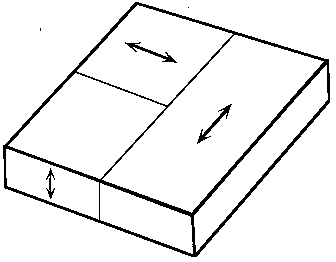
Figure 3
Note that, since BaTiO3 lacks a centre of symmetry in its low-temperature tetragonal form, each of these orientations actually corresponds to two possible orientations of the dipole vector. The sense of the dipole orientation cannot be distinguished by optical means.
When the thin slice of BaTiO3 provided in this demonstration is heated above 120 °C it becomes a single crystal of the cubic polymorph with the plane of the slice parallel to a face of the form {100}. On transforming to the low-temperature structure the possible domain orientations are as indicated in Fig. 3.
The heating stage provided (Fig. 4) comprises a metal block which is heated by a soldering iron. The temperature of the block is indicated by a mercury thermometer. A thin crystal of BaTiO3 is supported on a copper grid, which transmits heat from the block to the specimen.
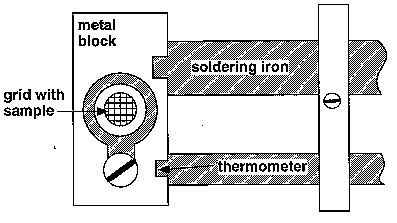
Figure 4
The polarising microscope contains two polars, known as the polariser and analyser. These cause the incident light to be polarised in one direction only; when the polariser and analyser are at 90
° to each other (with no specimen on the microscopic stage), no light can pass through the microscope. This condition is known as "crossed polars". In this experiment, the polariser is in a fixed position just below the microscope stage, whilst the analyser may be inserted perpendicular to this to the left-hand side of the eye–piece.Birefringent substances can change the state of polarisation of light; in other words, if a birefringent sample is placed between crossed polars, some light may be transmitted through the microscope because the state of polarisation of the light has been altered. BaTiO3 is such a substance, being isotropic in its high-temperature form, but anisotropic below 120 °C.
2.1 To observe the phase transition in BaTiO3 switch on the soldering iron and, when the temperature rises above 100 °C on the thermometer, watch the behaviour of the specimen carefully between crossed polars. When the specimen transforms it will become isotropic, at which point the soldering iron should be switched off. Transformation to the low temperature state occurs suddenly with falling temperature, and careful observation will often reveal a complex moving domain pattern in the specimen just before the transition temperature. It is possible, when the specimen is at a temperature just above the transition temperature, to cause it to transform by blowing on it very gently while at the same time watching its behaviour through the microscope. There should be ample time in the demonstration to cycle through the transformation a number of times.
3. X-ray powder diffraction of BaTiO3
A specimen of BaTiO3 held above 120 °C yields a simple cubic powder diffraction pattern, whereas in the diffraction pattern of a room temperature specimen many of the lines are split. This is attributed to the displacive phase transition at 120 °C, below which the unit cell is distorted to tetragonal with a = b but not equal to c. Diffractometer traces for the cubic and tetragonal phase are given in Fig. 5 (CuKa radiation was used).
3.1 The first ten diffraction peaks on the 320 °C diffractometer trace correspond to the following interplanar spacings:
|
4.012 |
2.837 |
2.316 |
2.005 |
1.795 |
1.638 |
1.420 |
1.336 |
1.270 |
1.209 |
Å |
Assign indices to the spacings, and add these indices to the corresponding peaks of the 320 °C diffractometer trace on Fig. 5. State the lattice type and determine the unit cell dimension.
3.2 The room temperature diffractometer trace is also depicted in Fig. 5 . Firstly, treat it as a cubic powder pattern, and give a value N = h2 + k2 + l2 to each group of peaks, ignoring the splitting caused by the slight cell distortion. This may be done by inspection, and by comparison with the results of 3.1.
3.3 Which planes are related to (100) by cubic symmetry? Hence determine the multiplicity of the 100 reflection in the cubic diffraction pattern. Are all of these planes related to (100) in the tetragonal structure? Which are not?
3.4 The powder peaks corresponding to the cubic form {h00}, i.e., 100, 200 etc., should thus each be split into two components, the separation of which increases with h in the room temperature diffraction pattern. Verify this on your diffractometer trace, and by considering the multiplicity values of {h00} and {00h} and their influence on the intensity of the corresponding diffraction peaks, assign the correct indices to each component of the split peaks. The interplanar spacings for the components of line N = 16 are 1.007 Å and 0.9963 Å; calculate values of the cell parameters "a" and "c".
3.5 Another possible distortion of the simple cubic cell causes a change to orthorhombic symmetry with approximately the same cell size. How could such a change be detected from the h00 peaks?
What would you expect to see on the diffractometer trace if, in addition, the distortion caused the c cell dimension to double in length?
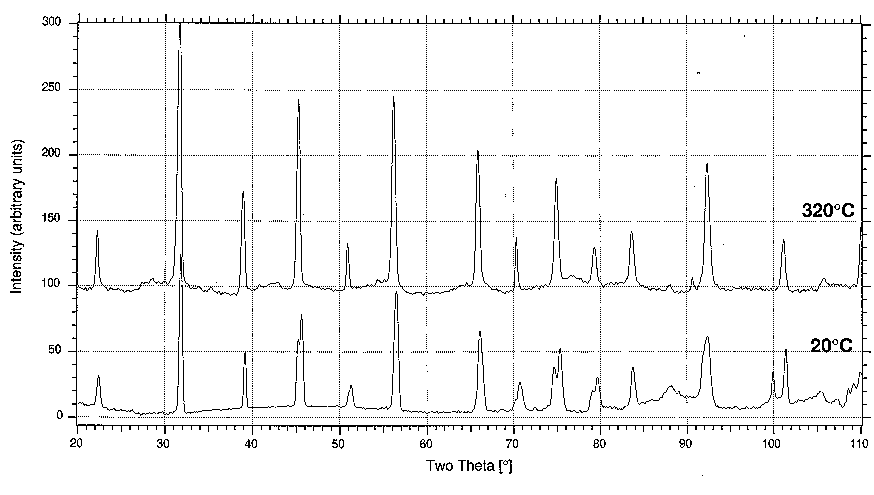
Figure 5
H. Bhadeshia & M. Dove, Easter Term 1999