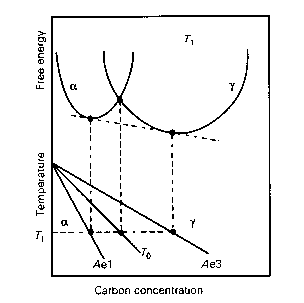
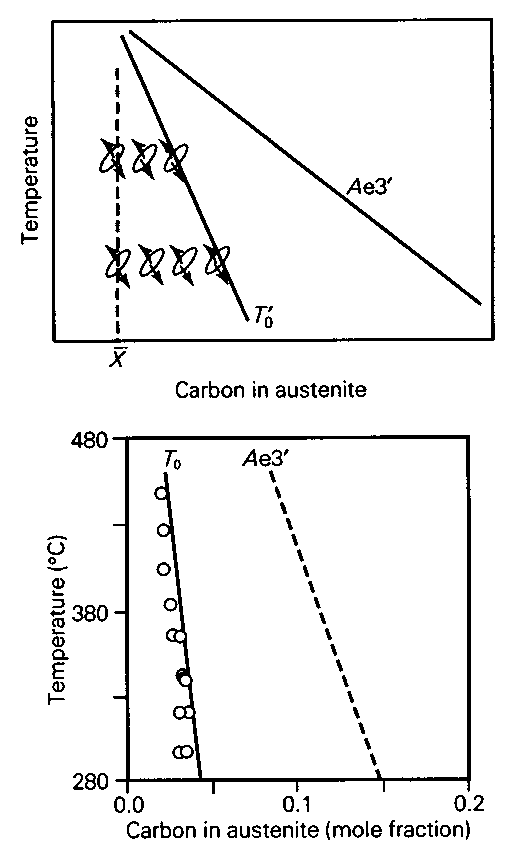
An illustration of the T-zero construction on the Fe-C phase diagram. Austenite with a carbon concentration less than that given by the T-zero curve can in principle transform without diffusion. But diffusionless transformation is not possible even in principle, if the austenite has more carbon than given by the T-zero curve. Alpha refers to ferrite and Gamma to austenite.