Transition metal dichalcogenide (TMD) compounds are promising catalysts for generating hydrogen from water. However, most TMD compounds possess low electrical conductivity so charge transfer required for the catalytic reaction occurs slowly. The poor charge transfer decreases the reaction kinetics and the amount of hydrogen produced.
We have successfully synthesized form of niobium disulfide that possesses very high electrical conductivity – close to that of metals. This highly conducting phase, first reported in 1960 but not extensively studied since then, contains excess niobium atoms in the usual structure of niobium disulfide. The metallic properties of this phase leads to substantial improvements in reaction kinetics allow production of hydrogen at unprecedented rates.
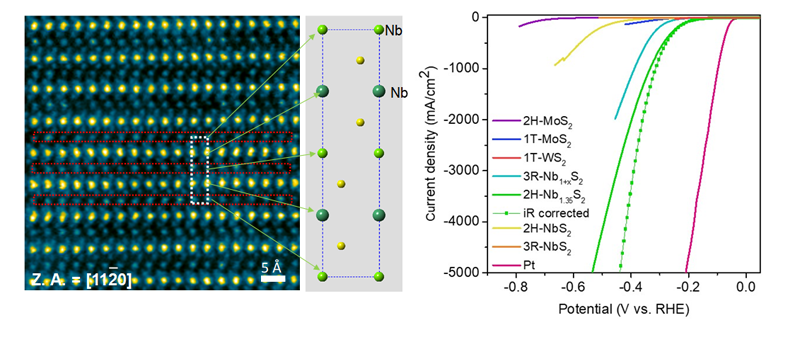
Figure caption: (Left) Cross-sectional ADF-STEM image of the niobium disulfide phase. (Right) Catalytic activity of different TMD compounds tested in our study. The current density of 2H Nb1.35S2 shows very high current density at low over potentials.
J. Yang, A. Mohmad, Y. Wang, R. Fullon, X. Song, F. Zhao, I. Bozkurt, M. Augustin, E. J. G. Santos, H. S. Shin, W. Zhang, D. Voiry, H. Y. Jeong & M. Chhowalla, "Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution", Nature Materials (2019)