Nanoparticles of gold, silver, and aluminium can capture light’s energy via localized surface plasmon resonances, an oscillation of the particle’s electron cloud. Once excited, this resonance can enhance the electric field of light as well as transfer energy to nearby molecules. This could potentially power chemical transformations or, even better, deliver energy to a molecule undergoing a catalyzed process, thereby promoting faster and more selective reactions.
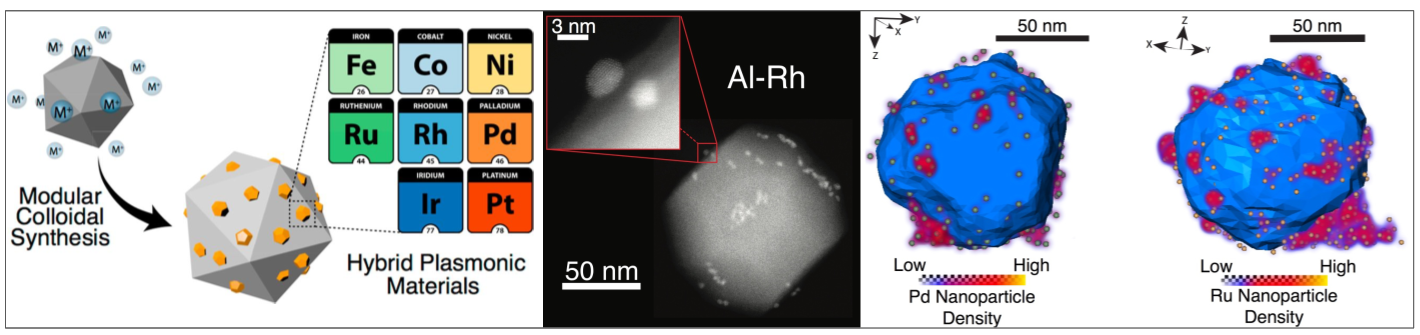
Bimetallic nanoparticles incorporating a core capable of localized surface plasmon resonances and a shell of a catalytic metal have been shown to increase selectivity and activity in a variety of reactions, however their synthesis remains a challenge. The group of newly appointed lecturer Emilie Ringe, in collaboration with Dr Rowan Leary, Prof. Paul Midgley (Cambridge) and Prof. Naomi Halas (Rice U.) developed a synthesis technique that uses aluminium nanocrystals as a base for size-tunable islands of transition metals, many of which are plasmonic. Electron tomography revealed the size and spatial distribution of the islands, revealing that most have nearby neighbors that could facilitate plasmon coupling and further enhance chemical reactivity.
Figure: Al nanoparticles were successfully decorated with eight transition metals using a non-aqueous, air-free reduction route. Electron microscopy (middle) and tomography (right) revealed the size and spatial distribution of the catalytic metal islands.
Swearer, D.F., Leary, R.K., Newell, R., Yazdi, S., Robatjazi, H., Zhang, Y., Renard, D., Nordlander, P., Midgley, P.A., Halas, N.J. and Ringe, E., "Transition Metal Decorated Aluminum Nanocrystals", ACS nano, 11 (2017) 10281-10288