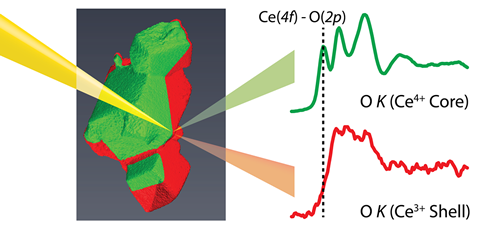
A new electron microscopy and data processing technique has been developed to reveal sub-nanometre changes in the chemistry at the surface of a cerium oxide-based catalyst. Cerium oxide catalysts, used widely in automotive catalysts for exhaust processing as well as other soot and chemical catalysis applications, are increasingly doped with other "rare earth elements" like lanthanum to improve their performance. The active chemistry occurs at the surface, however, which can be difficult to distinguish experimentally in three-dimensional nanoparticles of these catalysts. In this work, advanced algorithms and electron microscopy and spectroscopy techniques have been used to reveal how the bonding between atoms within 1.5 nm of the surface of these catalysts changes - in fully three-dimensional detail. The findings, that oxygen content is low at the surface and the lanthanum content is enriched, will aid in the engineering of improved, high-efficiency catalysts.
Figure: An illustration of electron microscopy measurements separating surface and bulk signatures of chemical bonding in cerium oxide-based catalyst nanoparticles.
Sean M. Collins, Susana Fernandez-Garcia, José J. Calvino & Paul A. Midgley, "Sub-nanometer surface chemistry and orbital hybridization in lanthanum-doped ceria nano-catalysts revealed by 3D electron microscopy ", Scientific Reports 7, Article number: 5406 (2017)