Under normal conditions the noble gases, helium, neon and the heavier argon, krypton, xenon and radon, are unreactive. One of the enduring geochemical mysteries is the apparent lack of xenon in the Earth's crust and atmosphere. It has long been speculated that xenon might be locked up in compounds under extreme compression within the Earth - but little is known about xenon compounds, or even whether they can exist.
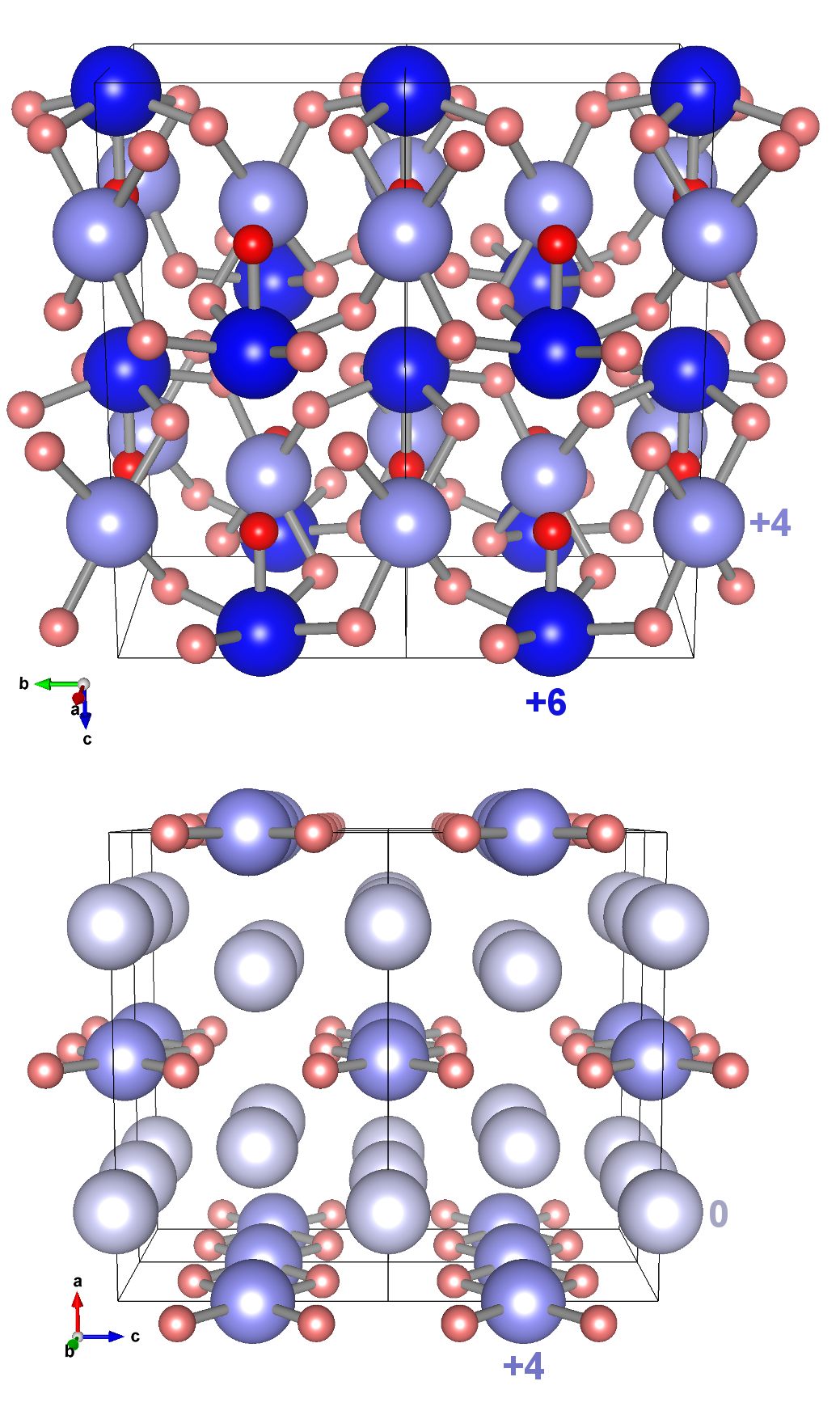
Combining theoretical structure prediction techniques, with diamond anvil cell high pressure experiments, two xenon oxides have been synthesised and characterised below 1 Mbar (100 GPa). The xenon adopts mixed oxidation states and forms extended networks that incorporate oxygen-sharing XeO4 squares. Xe2O5 additionally incorporates oxygen-sharing XeO5 pyramids. In combination with previous theoretical work, xenon's rich chemistry under extreme conditions is being revealed.
Figure: The crystal structure of the newly discovered xenon oxides, Xe2O5(top) and Xe3O2 (bottom). The oxidation states of xenon are labelled.
Agnes Dewaele, Nicholas Worth, Chris J. Pickard, Richard J. Needs, Sakura Pascarelli, Olivier Mathon, Mohamed Mezouar & Tetsuo Irifune, "Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure", Nature Chemistry (2016)