This course covers a modern, fast-growing and interdisciplinary area with very flexible boundaries. Great diversity arises in materials because they comprise atomic and molecular structures organised in complex patterns over many different length scales. The resulting intricate microstructures produce striking physical properties, leading to electrical, optical and mechanical behaviour of both scientific and technological relevance.
This course explores the fascinating science of structure-property relationships through an integrated system of lectures and practicals that are supplemented by web-based learning. You will engage in a wide range of hands-on activities, including nanoscale characterisation and fuel-cell construction. In addition, you will learn, for example, how liquid-crystal displays work, how biomaterials inspire materials design, and why aeroplanes do not fall apart. The course forms an important part of physical sciences teaching at Cambridge, and contains invaluable background knowledge to underpin in subsequent years, the study of Materials Science, Mineral Sciences or other physical sciences such as Physics and Chemistry.
- Informal prospective study enquiries may be made to the Director of Undergraduate Teaching (DUT@msm.cam.ac.uk).
- Information for current students is found on the relevant Moodle course.
This page is for external visitors and gives general information on the lectures and course activities which are available during the 2023-24 academic year. Please be aware that the lectures and activities offered can change from one year to the next, as may the lecturers who deliver them.
A: Atomic Structure of Materials Prof J A Elliott (Michaelmas Term, 6 lectures)
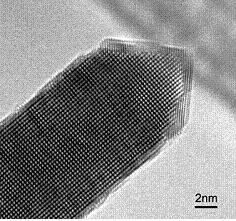
The atomic structure of any material is the key factor in understanding its physical and chemical properties. In principle by knowing where the atoms are, and what they are, then any property can be determined. Moreover, by manipulating the position of the atoms or substituting one atom for another then the properties of the material can be altered in a dramatic fashion - such design at the atomic, and microstructural, level is the key to modern materials science. In this course we investigate the arrangement of atoms in materials, the periodic structure and symmetry of crystals and how best to describe the crystal structure.
B : Materials for Devices Prof B Monserrat (Michaelmas Term, 12 lectures)
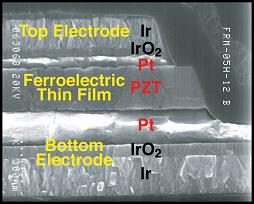
This second course begins with an introduction to polymers, and the fascinating phenomena shown by liquid crystals. Somewhere between the disordered structure of liquids and the very ordered structure of crystals, these stiff polymer molecules show different types of alignment, or directional ordering, leading to optical effects which are exploited in display technology. Picking up on the importance of crystalline structure, as covered in Course A, we will then go on to learn how the structure of a material defines its physical properties, with examples of practical applications: Why are some materials magnetic? How can we make use of the electrical polarisation exhibited by others? How can electrical and magnetic properties be linked in device structures? This course will consider the origin of ferroelectric, pyroelectric and piezoelectric effects, and describe how they are exploited in a range of device applications. The technological importance of electrical transport by solid ion conduction is also discussed, with reference to sensors and fuel cells.
C Diffraction Dr N A Rutter (Michaelmas Term, 6 lectures)

Diffraction techniques are essential tools in the investigation of the structures of materials. This course will cover the fundamentals of diffraction and imaging by considering the wave behaviour of light, X-rays and electrons. Beginning with light, the basic concepts of diffraction and interference will lead us to conclusions about the operation of optical microscopes, and explain why the resolution of such microscopes is limited to the micron-plus length scale. We will then consider X-rays, an ideal radiation source with which to investigate the atomic-scale structures of solids, looking at how X-rays are produced, how they interact with matter and how the diffraction patterns formed can be detected and interpreted. Finally we will explore how electrons (considered as waves) can provide excellent resolution and are also able to be focussed in to an image using electromagnetic lenses, as the course concludes with an introduction to electron microscopy.
D: Microstructure Prof S Kar-Narayan (Lent Term, 12 lectures)
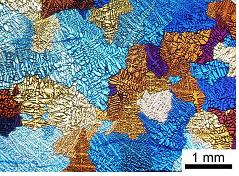
The properties of any material are critically dependent upon its internal microstructure, as well as the arrangement of atoms in the phases present. This microstructure may consist of a 'simple' grain structure, or more complex, multiphase components. Examples include precipitates in the form of aligned platelets, 'tree-like' growths (called dendrites), and regular plates of alternate phases - all of which may exist on length scales ranging from a few atomic spacings to many tens or hundreds of microns. In this course, microstructure formation is explained, based on thermodynamic and kinetic factors. The effects involved are illustrated with reference to metallic alloys and ceramic materials, and an introduction is given on how microstructural control in such systems can allow fine-tuning and optimisation of material properties.
E: Mechanical Behaviour of Materials Dr D Collins (Lent Term, 12 lectures)
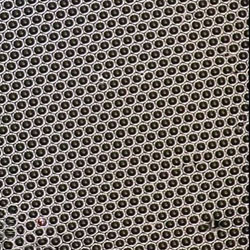
The mechanical properties of structural materials are dependent on the nature of the interatomic interactions, the crystal structures of the phases that are formed, the microstructures that they adopt and, critically, on the presence of defects. By understanding the phenomena that limit the strength of structural materials it is possible for us to get the very best performance we can from them. This course covers the basics of stress, strain and elasticity; the occurrence of dislocations in crystal structures and their role in plastic deformation; mechanisms by which we can strengthen materials; and, includes an introduction to fracture mechanics.
F: Biomaterials Prof A L Greer (Easter Term, 6 lectures)
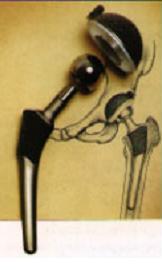
Living systems are composed of materials that, through evolution, have properties remarkably well adapted for their function, as assessed using materials-selection charts. Spider silk, for example, shows outstanding elastic behaviour, while wood is an excellent structural material. Living systems regulate phase transformations, notably the nucleation and growth of crystals, to ensure their survival and to build hard tissues such as shells and bones. In this course, we examine such topics, noting how the hierarchical structure of biomaterials can provide inspiration for the design of man-made materials. We also look at the opportunities and challenges of using man-made materials in the body, for example as implants such as hip prostheses (figure).
G: Materials Under Extreme Conditions Prof J H Gwynne (Easter Term, 6 lectures)
The ultimate properties of materials, tested under extreme conditions, are of great fundamental interest, relating closely to structure and bonding. Most technologies are limited by the materials used, so the ultimate properties are of practical importance also. In this course, we look at a range of extreme conditions. We consider the effects of high stress and high temperature, leading to the particular example of turbine blades in jet engines, and we analyse the design of alloys to resist slow deformation (creep). We also consider materials under irradiation, focusing on the stability of their structure. At very small sizes, the properties of materials are influenced by their surfaces as well as their bulk: this is the world of nano-science and technology. We examine some properties of materials at the nanoscale.
