The discovery of new materials, or innovative uses of existing materials, is essential to making progress in many of the technological challenges we face: from collecting energy from the sun to interfacing with the human body. Research in the department ranges from the chemical synthesis of new compounds and design of superalloys, to theoretical modelling and even the computational prediction of new materials. Recent highlights include the design and synthesis of non-toxic photovoltaic materials, high temperature superalloys, discovering a way to make brittle materials plastic and a new family of glasses, biomedical scaffolds and a theoretical understanding of a record breaking superconductor.
Hybrid glasses (Bennett)
Glasses are ‘frozen liquids’, formerly thought to belong to three categories: inorganic, organic or metallic. The most common route to the glassy state involves melt-quenching, i.e. cooling a liquid on a timescale fast enough to avoid molecular or atomic reordering to an ordered state. On heating, glasses undergo a reversible transition to a softer, more liquid-like state at their glass transition temperature (Tg).
Despite the enormous number (>80,000) of crystalline hybrid solids such as MOFs and HOIPs known, their disordered states remain almost totally unknown. This group have pioneered the synthesis and characterisation of glasses formed by melting hybrid solids. For example, the melt-quenching of several zeolitic imidazolate frameworks results in glasses which retain the metal-organic-metal connectivity of the crystalline state, though are bulk, transparent, grain-boundary free materials. Equally, we have also shown that hybrid perovskite materials are also glass formers, and that the glasses display interesting thermoelectric properties.
The glasses belong to the family of hybrid glasses – a new 4th category of glass. Their structural, mechanical, chemical and optical properties are thus of extreme interest, and the group works on building structure-property relationships so that we can design the next generation of functional glass.
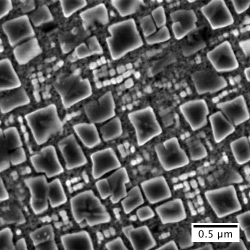
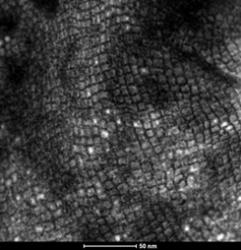
The drive for more efficient civil air transportation requires the next generation of jet engines to operate at higher temperatures. Current materials are already operating at their limit and as a result it is critical to develop new materials with higher temperature capability. Research in the department is taking place to develop a range of new materials, including Ni-base and Co-base superalloys, High Entropy Alloys and Refractory Metal-base alloys. The approach combines empirical relationships, thermodynamic and first principles calculations, as well as neural networks to identify promising candidate materials prior to evaluation through rapid small scale alloy prototyping. An electron image of a novel Ni-based superalloy developed in the Department is shown.
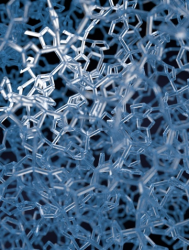
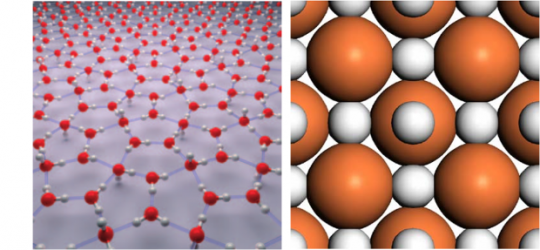
The combined power of modern computers and robust first principles codes has made computational materials discovery possible. Ab initio random structure searching (AIRSS) can be used to predict crystal, defect or grain boundary/interface structure, with little or no experimental input. Combining this with finite temperature calculations, materials can be discovered and optimised for applications in technological devices. Recent applications include the discovery of two dimensional ice structures (top), and understanding the crystal and electronic structure of the record-breaking (Tc=204K) superconductor, hydrogen sulphide at high pressure (bottom).
Plasticity in brittle materials
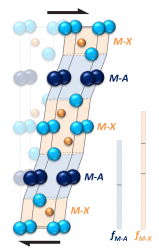
A major difficulty in developing materials for use at high temperatures is that in most oxidation resistant materials, the predominant obstacle to dislocation motion is due to the changes in misfit energy as a dislocation moves, causing them to be brittle. At present, there is virtually no understanding of how to design brittle crystals to give easy plastic flow. Using density functional theory calculations, it has been found that small modifications to the electronic structure and elastic deformation of crystals can drastically affect plastic flow. This appears to be a general method by which dislocation properties may be controlled to allow easier flow and this concept is consistent with observations in crystals. Furthermore, it is a substantial effect suggesting that such an approach might be used as a general way of tailoring plasticity in crystals. Deforming a MAX phase crystal.
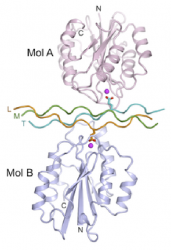
In order to direct cell adhesion and proliferation, the chemistry of biomedical scaffolds with three dimensional porous architectures is being controlled using biochemical surface modification. Triple helical peptide (THP) sequences are used to mimic defined functions. These peptides have been synthesised by collaborators in the Department of Biochemistry. Using the high integrin affinity ligand, GFOGER, cell reactivity has been shown to be promoted. The mechanism for integrin binding to peptide sequences in collagen is shown.
Organic-inorganic hybrid polymers (Evans)

Class II organic-inorganic hybrids combine the best of both worlds: chemical functionality and elasticity from the organic polymer and optical transparency from the inorganic part (e.g. silica, zirconia). We are developing new class II hybrid materials with tuneable physicochemical and optical properties (see picture) for a diverse range of applications including spectral conversion for solar cells (upconversion and downshifting), optical sensing and membrane technology.
Nanoparticle synthesis (Evans)
We are investigating a variety of methods (sol-gel, reprecipitation, emulsion) to fabricate diverse photoactive nanomaterials for application in light-emitting displays (e.g. perovskite nanocrystals – see image), catalysis and environmental remediation (e.g. porous inorganic oxide nanoparticles from stimuli-responsive templates) and optical sensing/theranostics (e.g. organic-inorganic hybrid polymers).