Professor of Materials Chemistry
MSci University of Cambridge
PhD University of Cambridge
Metal-Organic Frameworks: Crystalline Solids, Gels, Liquids and Glasses
Crystalline Solids
Metal-organic frameworks (MOFs) are network solids in which inorganic nodes (clusters or metal ions) are linked via organic ligands in an infinite array. The synthesis of novel MOF materials has been the subject of intense research and debate over the last decade, mainly because of their potential for application in gas storage and separation, catalysis and chemical sensing. Over 70,000 structures now exist, with several commercial uses in toxic gas storage, the prevention of fruit over-ripening and vehicular hydrogen storage.
Mechanical properties such as the bulk, shear and Young’s modulus, alongside the hardness and thermal expansion of materials are incredibly important for their industrial applications. The group have expertise in the use of variable temperature powder X-ray diffraction, high pressure powder X-ray diffraction and nanoindentation to elucidate the properties of crystalline metal-organic framework materials.
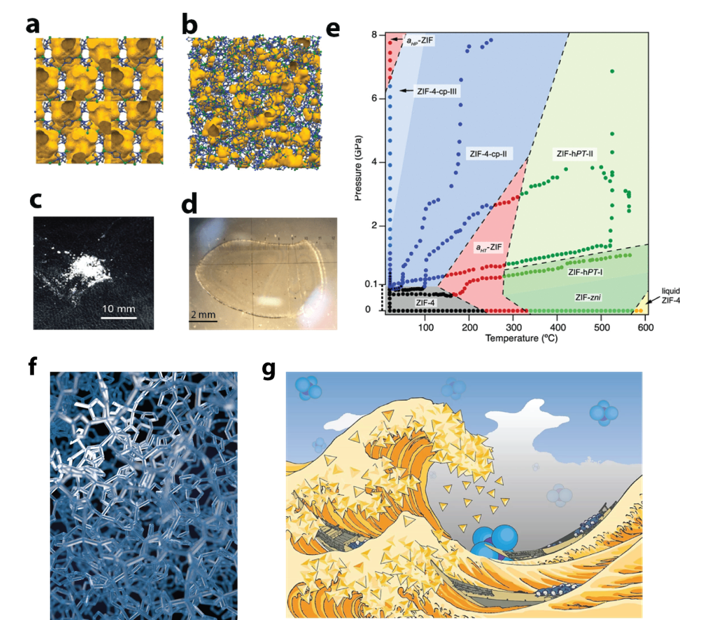
MOFs Under ‘Extreme Conditions’
The group have produced the first high-pressure high-temperature phase diagrams of MOFs. The study of MOFs under ‘Exotic Conditions’ such as those found beneath the Earths crust, have in some cases revealed the existence of new structures, and in others demonstrated unusual physical phenomena such as a negative melting curve.
Metal-Organic Framework Gels and Monoliths
The group has worked extensively on the synthesis and structure of metal-organic framework gels. Particular, our interest lies in the formation of MOF nanoparticles of ca. 10nm in diameter, which then agglomerate upon drying to form xerogels, or aerogels. These peculiar structures to all intensive purposes look like glasses, though are in actual fact still crystalline (though the broad Bragg peaks may resemble the diffuse scattering from a glass).
Hybrid Glasses
Glasses are ‘frozen liquids’, formerly thought to belong to three categories: inorganic, organic or metallic. The most common route to the glassy state involves melt-quenching, i.e. cooling a liquid on a timescale fast enough to avoid molecular or atomic reordering to an ordered state. On heating, glasses undergo a reversible transition to a softer, more liquid-like state at their glass transition temperature (Tg).
Despite the enormous (>70,000) crystalline hybrid solids known, the glassy state is almost totally unknown. The group have pioneered the synthesis and characterisation of glasses formed by melting hybrid solids. For example, the melt-quenching of several zeolitic imidazolate frameworks results in glasses which retain the metal-organic-metal connectivity of the crystalline state, though are bulk, transparent, grain-boundary free materials. Equally, we have also shown that hybrid perovskite materials are also glass formers, and that the glasses display interesting thermoelectric properties.
The glasses belong to the family of hybrid glasses – a new 4th category of glass, Their structural, mechanical, chemical and optical properties are thus of extreme interest, and the group works on building structure-property relationships so that we can design the next generation of functional glass.
Glass-Based Composites
The group are interested in exploiting the liquid and glass states as reactive components in the formation of more complex bulk structures. For example, MOF glasses have been used as hosts for secondary crystalline MOF structures, and shown to be capable of stabilising highly porous crystalline materials which are unable to exist in the pure state at room temperature.
We have however also shown that two liquid MOFs may be ‘blended’ together, as in organic polymers, and that the strategy can be used to exert control on the resultant physical properties of the glass blends formed. The structural complexity of such materials however require advanced structural characterisation techniques, which probe not only the components themselves, but also the interfaces between them.
Functional Liquids
The group uses advanced experimental techniques to investigate the mechanism by which crystalline hybrid solids melt, and probe the structure of the advanced liquids produced. The use of variable temperature total X-ray scattering measurements, alongside differential scanning calorimetry, yields valuable information on the dynamic structure of these liquids. Work has concentrated on the formation of room temperature porous liquids for the capture of CFCs, and on the mechanism by which zeoilitic imidazolate frameworks melting.
- ‡ L. Ma, A. B. Grommet, C. J. E. Haynes, A. Walczak, C. C. Parkins, L. Longley, A. Tron, T. D. Bennett* and J. R. Nitschke, "Coordination cages as a scaffold for permanently porous liquids", Nat. Chem., 2020, 12, 270-275
- J. Hou, C. W. Ashling, S. M. Collins, A. Krajnc, C. Zhou, L. Longley, D. N. Johnstone, P. Chater, S. Li, M. V. Coulet, P. L. Llewellyn, F. X. Coudert, D. A. Keen, P. A. Midgley, G. Mali, V. Chen, T. D. Bennett, "Metal-organic framework crystal-glass composites", Nat. Commun., 2019, 10, 2580
- R. N. Widmer, G. I. Lampronti, S. Anzellini, R. Gaillac, S. Farsang, C. Zhou, A. M. Belenguer, H. Palmer, A. K. Kleppe, M. T. Wharmby, S. A. T. Redfern, F. X. Coudert, S. G. Macleod, T. D. Bennett, "Pressure promoted low-temperature melting of metal-organic frameworks", Nat. Mater., 2019, 18, 370-376
- T. D. Bennett* and S. Horike' "Liquid, glass and amorphous solid states of metal-organic frameworks", Nat. Rev. Mater., 2018, 3, 431-440
- † R. Gailliac, P. Pullumbi, K. A. Beyer, K. W. Chapman, D. A. Keen, T. D. Bennett, F. X Coudert, "Liquid metal-organic frameworks", Nat. Mater., 2017, 16, 1149-1155