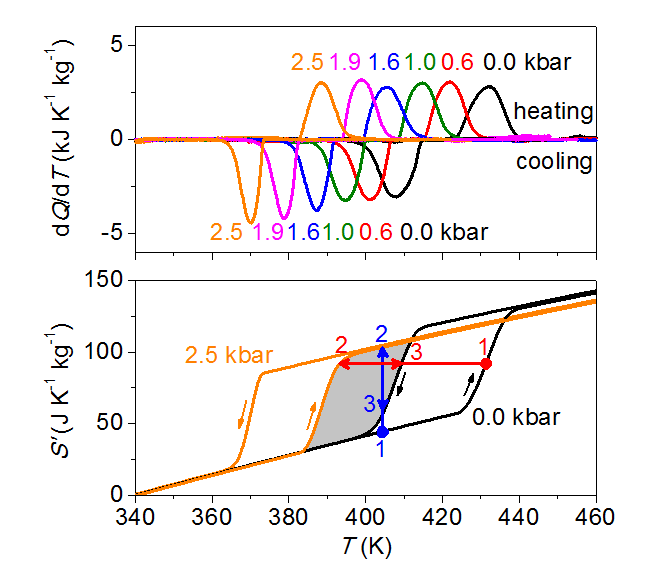
First-year undergraduates in this department perform an experiment in which they increase the electrical conductivity of silver iodide by melting the cation sublattice. Given that this thermally driven entropic transition is accompanied by large changes of volume, it can instead be driven by changes of applied pressure. The resulting barocaloric effects that we have recently reported represent an improvement with respect to state-of-the-art barocaloric materials, both in the isothermal limit where the peak entropy change is 0.34 J K‑1 cm‑3, and in the adiabatic limit where the peak temperature change is 18 K. These peak values fall only slowly on varying the start temperature, as required for effective operation in prospective heat pumps. The discovery of large barocaloric effects in a solid electrolyte should inspire the development of cooling devices based on such materials, as well as the discovery of large barocaloric effects in other solid electrolytes.
Figure: The upper panel shows how the melting transition associated with the cation sublattice is modified by pressure p, permitting the barocaloric effects shown in the lower panel to be identified. (Barocaloric effects are thermal changes that arise due to changes of pressure, Q is heat, T is temperature and S' denotes the entropy with respect to the zero‑pressure entropy at our base temperature of 340 K.)
A. Aznar, P. Lloveras, M. Romanini, M. Barrio, J.-Ll. Tamarit, C. Cazorla, D. Errandonea, N. D. Mathur, A. Planes, X. Moya, and Ll. Mañosa, "Giant barocaloric effects over a wide temperature range in superionic conductor AgI", Nature Communications 8, 1851 (2017).