This course applies a broad range of scientific principles in the quest to design new, more advanced materials as well as to understand the functions of materials in modern society. Hence the course is of great benefit to anyone intending to pursue Materials Science, Physics or Chemistry at a higher level. Some ideas introduced in the IA course are studied in much greater depth in order to produce a thorough understanding of how the key scientific aspects of processing, structure and properties interact with factors such as cost, safety and sustainability when considering which materials are suitable for particular applications.
The course looks at advances that continue to be made with metallic materials, where new developments continue to drive forward the improvements in properties of metallic alloys, and also in the area of polymers and other “soft materials” such as liquid crystals. Alongside this, you will look at how materials function in service, whether a material it is likely to degrade through chemical processes and when a structure may be susceptible to failure under the imposed mechanical forces. In addition, you will learn the scientific principles of functional materials, such as semiconductors, that have revolutionized society in the last few decades, allowing us to build smaller and more powerful devices by combining new materials with advanced fabrication processes. In addition to the lecture courses and associated practicals, there are visits to materials-related companies and also a project which involves dismantling and examining a household item.
- Informal prospective study enquiries may be made to the Director of Undergraduate Teaching (DUT@msm.cam.ac.uk).
- Information for current students is found on the relevant Moodle course.
This page is for external visitors and gives general information on the lectures and course activities which are available during the 2023-2024 academic year. Please be aware that the lectures and activities offered can change from one year to the next, as may the lecturers who deliver them.
A: Metals and Alloys Prof H J Stone (Michaelmas Term, 12 lectures)

Improving metallic materials is a vital activity at the leading edge of science and technology. Metals have offered unrivalled combinations of properties and reliability over many centuries, generally at affordable costs. They are versatile because subtle changes in their microstructures can cause significant variations in their properties, e.g. the strengths of commercial steels range from as low as 50 MPa to up to 5500 MPa. It is possible to specify and produce metals with very specific properties and so metals continue to be used in many different applications despite the increased competition from other classes of material such as polymers, ceramics and composites. Hence an understanding of the development of microstructure in metals is essential for the materials scientist. The course builds on the coverage of metals and alloys in Part IA. Whereas Part IA dealt mainly with thermodynamic aspects, kinetics are emphasised when treating phenomena such as diffusion, solidification and solid-state transformations.
B: Mechanics of Materials and Structures Dr R P Thompson (Michaelmas Term, 12 lectures)

Any structure, whatever its function, must be able to withstand the loads imposed upon it during its lifetime. While mere survival may sometimes be enough, often such loads must be carried efficiently. Building on ideas introduced in Part IA, this course aims to understand the approaches that can be used to enable materials and structures to withstand stresses efficiently by considering both the nature of the stresses and how materials respond to them both elastically and irreversibly, illustrating these concepts using both synthetic and natural materials and structures.
C: Materials Chemistry Prof T D Bennett (Lent Term, 9 lectures)
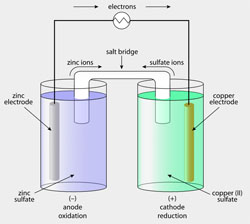
Almost all materials in service are degraded by chemical reaction with their environment. The phenomenon is called corrosion; it is very often corrosion which limits the useful lifetime of components. The process is extremely costly - apart from replacement costs of components, equipment and machinery, corrosion causes failure, sometimes catastrophic, resulting in injury and loss of life. Corrosion or environmental degradation consumes materials, and the process is thus a drain on the world's mineral resources and energy resources.
The phenomenon takes many forms, depending on the material/environment system. The first part of this course focuses on the corrosion and oxidation of metals, dealing with how and why metals corrode, how we can predict, measure and finally, prevent the processes.
D: Structure and Characterisation Prof C J Pickard (Lent Term, 6 lectures)
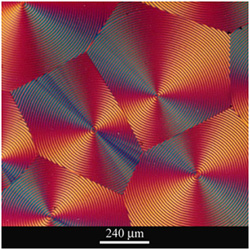
In this short course of 6 lectures we look at a number of techniques that enable a detailed characterisation of materials at the nano- and micron-scale. We start with a brief revision of some of the key aspects of crystallography and diffraction before moving on to how x-ray diffraction can be used to determine not only the atomic structure of crystals but can also provide important information about the microstructure (e.g. strain, grain size, texture, thin films). A large fraction of the course is focussed on how microscopy can directly reveal microstructures, firstly using optical microscopy (e.g. bright and dark-field imaging, polarization microscopy, interference contrast) to reveal features at the micron scale and secondly scanning electron microscopy that provides structural and chemical information at the nanoscale. The final part of the course considers different kinds of spectroscopy (sometimes coupled with microscopy), such as X-ray or Raman spectroscopy, that can provide complementary information across a range of lengthscales.
E: Non-Metallic Materials Prof J H Gwynne (Lent Term, 9 lectures)
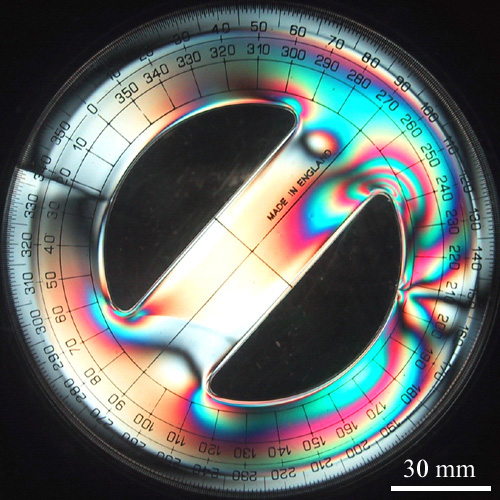
In this course, we will cover non-metallic materials, including glasses, ceramics and polymers.
The first half of the course concerns glasses and ceramics. We will begin with glasses, by looking at their structure, viscosity, crystallisation and processing. We will then cover processing methods for ceramics, including powder packing, hot isostatic pressing and slip casting, before moving on to their properties. We finish the first half with a short section about construction materials, including cement and concrete.
The second half of the course concerns polymers and elastomers. We will begin by revising and expanding on some concepts covered in Part IA, including conformation and configuration. We will cover different ways of defining and measuring molar masses, before moving on to different polymerisation methods (step-growth and chain-growth). In a section about amorphous polymers, we will cover the glass transition temperature, rubber elasticity, and crystallisation.
This course ties in with practical P7 in Michaelmas term, in which we will look at different polymer identification tests (designed to help with your Materials Manufacturing Project), and practicals P10 and P11 in Lent term, in which we will make and test some concrete, and investigate the fabrication and mechanical properties of polymers and elastomers.
F: Electrical Properties of Materials Dr P Chen (Easter Term, 8 lectures)

Electronic materials are exploited in a vast number of products, ranging from the components of the basic electrical circuitry providing power and lighting to homes and offices, to laptop computers, televisions and mobile phones. It is one of the fastest-developing areas - components are becoming smaller and smaller, and computers are becoming more and more powerful.
In this course, we will begin by covering some of the different models that have been used to explain electronic conductivity: a simple classical model (the Drude Model), and two quantum mechanical models (the Free Electron Model and the Nearly Free Electron Model). We will then look at the electrical properties of metals and alloys and briefly introduce superconductivity. The last part of the course concerns the properties of semiconductors and semiconductor devices.
