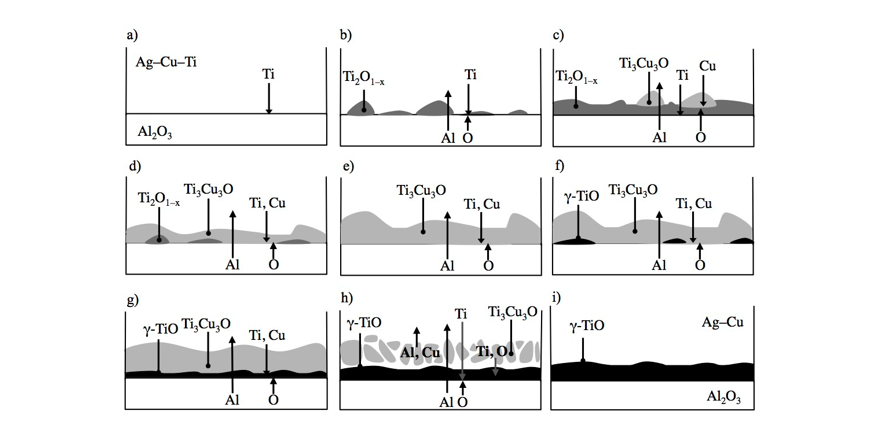
Banner image: a-f) Schematic mechanism for the evolution of a series of metastable interfacial phases at a Ag–Cu–Ti/sapphire interface during brazing, such as Ti2O1–x and Ti3Cu3O, g-h) and their disappearance so that γ-TiO is left as the most significant interfacial phase for overlong joining times, such as 10 h, i). Majed Ali, Kevin M. Knowles, Phillip M. Mallinson, John A. Fernie, “Interfacial reactions between sapphire and Ag–Cu–Ti-based active braze alloys”, Acta Materialia, 103, (2016), 859–869.
Structural ceramics is a research area in which the physical and mechanical properties of engineering ceramics are studies. Engineering ceramics differ from ceramics used for pottery in that they are used in practical applications which exploit properties such as their very high temperature capability, their high wear resistance and their ability to withstand thermal shock.
Examples of structural ceramics are refractory ceramics such as alumina (Al2O3) which are used as crucibles, silicon nitride ceramics used as cutting tools and cordierite-mullite refractories used for their good thermal shock behaviour.
Work here at Cambridge over the years has looked at a number of aspects relevant to structural ceramics such as how to join them to themselves and to other materials such as metals by active metal brazing so that the resultant component can be used at temperatures > 400 °C, i.e., temperatures above which ordinary adhesive glues are able to operate.
Active metal brazing is a relatively simple and versatile technique to join a wide range of ceramics to themselves or to metals. Braze filler alloys based on the Ag–Cu–Ti system have been used extensively to bond Al2O3 to itself. Although there is extensive scientific literature available on the interfacial structures between Ag–Cu–Ti-based alloys and Al2O3, the reaction processes responsible for the evolution of the interfacial phases, and ultimately the formation of strong bonds, have not been fully understood until our recent work in this area.
Using a range of electron microscopy-based techniques we have shown that when the Ag–Cu–Ti-based alloy melts, Ti first reacts with the Al2O3, dissolving significant amounts of oxygen, to form a transient reaction layer that enables the braze to wet the Al2O3. This reaction layer breaks down within tens of seconds to support the growth of Ti3Cu3O particles that nucleate behind it and are in contact with the liquid braze. Particles of γ-TiO are found to be the last interfacial phase to form in the joint. At extended bonding times, much longer than would be used in an actual brazing operation, γ-TiO becomes the only interfacial bonding phase. The detailed experimental observations as a function of bonding temperature in this work have been used to establish a definitive bonding mechanism for the joining of high purity Al2O3 with Ag–Cu alloys activated by small amounts of Ti.
